

Current projects
Completed projects
Research funding
Current Projects
Mechanisms of locomotor rhythm generation in rodent spinal cord
NIH/NINDS R01 NS130799; 09/30/2022 - 07/31/2027
PIs: Dougherty KJ, Rybak IA
Locomotion is a fundamental behavior that allows humans and animals to move through their environments and is critically involved in all aspects of life. This behavior is impeded in a number of diseases, disorders, and injuries, including spinal cord injury, stroke, and various ataxias. All of the essential circuity to generate locomotor rhythm and pattern is located in the thoracolumbar spinal cord, most often below the level of neural damage. These circuits can be accessed directly via various central and peripheral stimulation methods, including but not limited to epidural stimulation. Rhythm generating circuits are the entry point for initiation and control of locomotion, affect all downstream neurons related to locomotion, and, therefore, are the first step in establishing spinal control of locomotion. Successful activation of the rhythm generator clinically has been hampered because the mechanisms by which spinal neuronal circuits generate coordinated rhythmic output remain poorly understood and represents a major gap in our understanding of neural control of movement. The generation of rhythmic motor behaviors is based on a triad involving: (1) specific rhythmogenic properties allowing individual neurons to generate rhythmic oscillations, (2) mutual excitatory interactions to synchronize neuronal activity into rhythmic populational bursting, and (3) network inhibition to coordinate activity between different neuronal populations, which can both shape locomotor pattern and control frequency. Triad components are highly interconnected and the involvement of each component is condition-dependent. The proposed study will use highly integrated electrophysiological, pharmacological, genetic, and computational approaches to systematically explore the specific contributions of these mechanisms and the interactions between them, in the generation and patterning of the locomotor rhythm. Utilizing spinal neurons identified in transgenic mice by the transcription factor Shox2 as a representative rhythm generating population, we will test the overarching hypothesis that rhythm generating mechanisms in the spinal cord involve interplay between the triad of cellular, population, and network properties, whose contribution to rhythmogenesis is interdependent, leading to flexibility and adaptability seen as alterations in the relative balance of the triad in different conditions. We will first determine the voltage-gated currents underlying spontaneous cellular oscillations in adult Shox2 neurons. We will then assess excitatory interactions between rhythm generating neurons. Lastly, we will establish the role of ipsilateral and contralateral network interactions in regulating locomotor frequency and determine the operation of these pathways during afferent-evoked locomotion. Together, our multidisciplinary study will reveal mechanisms of rhythm generation, establish the first mammalian locomotor neural network model based on real rhythm generating cellular and network properties, and determine the ways by which afferent stimulation may influence the locomotor rhythm and pattern generated in the spinal cord. The results of these studies will identify specific neural targets for the future devices and strategies aimed at restoration of locomotion following injury or motor disorders.
CRCNS US-French Research Proposal: Brainstem-spinal circuits for control of locomotor steering
NSF 2113069; 10/01/2021 - 09/30/2025
PIs: Ausborn J, Bouvier J; Co-PI: Rybak IA
The ability to move within the environment is essential for the survival of all animals, including humans. In mammals, the neurons that generate and drive locomotor movements reside in the spinal cord, and these spinal networks are controlled by upstream signals from the brainstem. Most studies of neural control of locomotion in mammals have focused on straight-trajectory forward locomotion. However, how brainstem and spinal neural networks control turning movements remains poorly understood. This study will investigate this question, using a combination of experimental and computational approaches. This international CRCNS project will be performrd in close colllaboration between Dr. Julien Bouvier (Paris-Saclay University, France) and Drs. Jessica Ausborn and Ilya Rybak (Drexel University). The results of this project will provide important insights into the neural control of turning and, more broadly, the neural control of locomotion. The models developed in this project can serve as test-beds for simulating different aspects of motor disorders and treatment approaches. Study outcomes can help in the development of novel strategies to restore locomotor function after spinal cord injury, neurodegenerative pathologies, and other motor disorders. This multidisciplinary project will investigate the neural control of locomotion in mice with a focus on mechanisms of turning movements. A recently uncovered population of reticulospinal neurons in the gigantocellular reticular nucleus (Gi) of the brainstem projects to all segments of the spinal cord and is defined by the expression of the transcription factor Chx10 (V2a neurons). Experimentally activating these neurons in the mouse induces robust turning movements that seem to be mostly driven by an asymmetric control of the motor circuits of the neck, upper trunk, and forelimbs. This study will test the hypothesis that these pathways represent a major orchestrator of locomotor turning maneuvers. This study combines state-of-the-art physiological, genetic, pharmacological, and motion tracking approaches with computational modeling of the brainstem and spinal cord circuits and animal biomechanics. The results of in vitro and in vivo studies will be incorporated in a neuro-biomechanical data-driven model of quadrupedal mammalian (mouse) locomotion. The model will provide mechanistic explanations and generate testable predictions that will then be verified experimentally. The project has the following three objectives. (1) Study the influence of reticulospinal V2a Gi neuron activation on spinal circuits potentially involved in locomotor steering behaviors and computational modeling of these brainstem-spinal pathways and circuits; (2) Characterize kinematics of mouse locomotion during changes of locomotor direction and develop a full-body neuro-biomechanical model of mouse locomotion; (3) Study the role of different populations of reticulospinal V2a Gi neurons in locomotor steering and test model predictions, challenge model assumptions, and investigate general mechanisms.

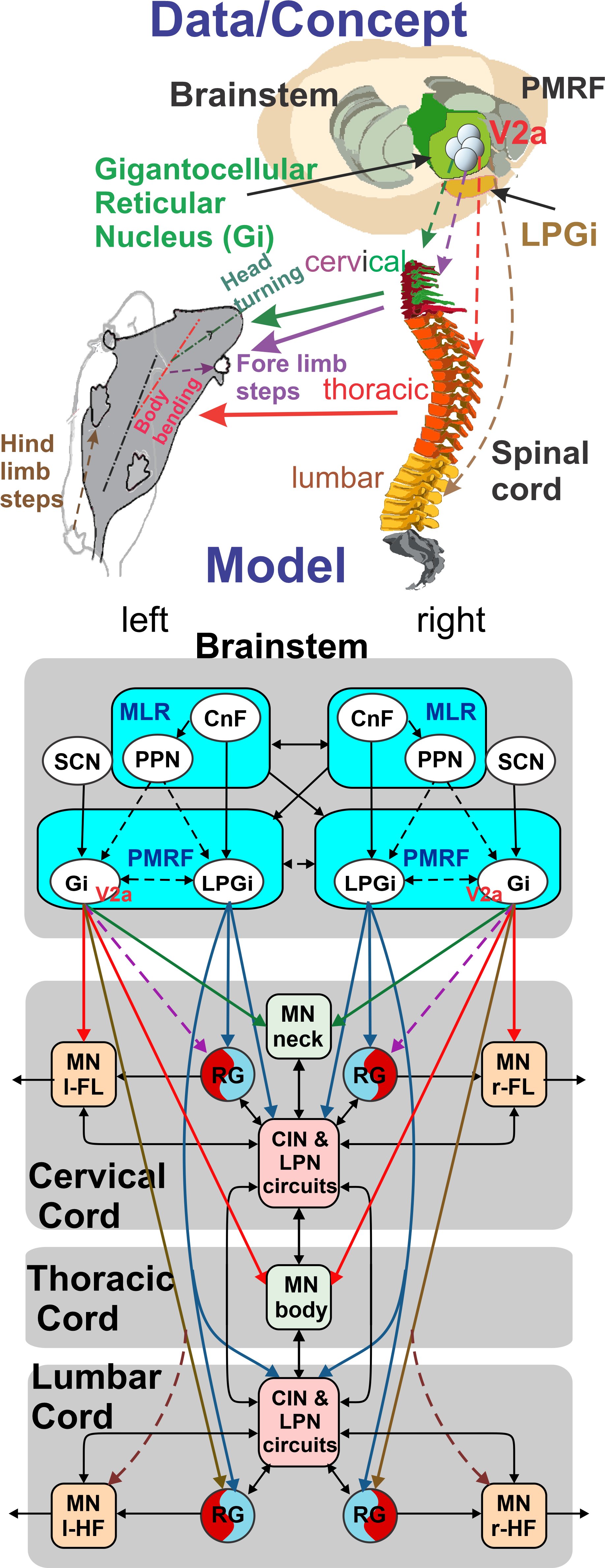
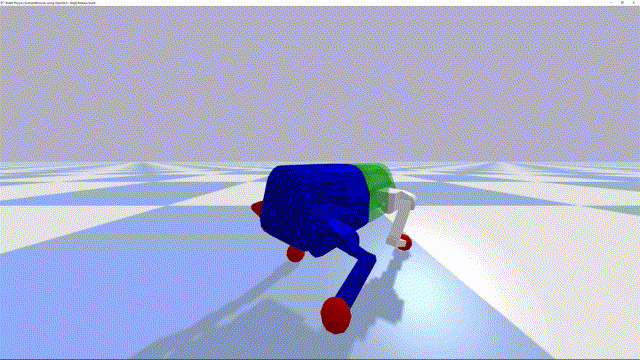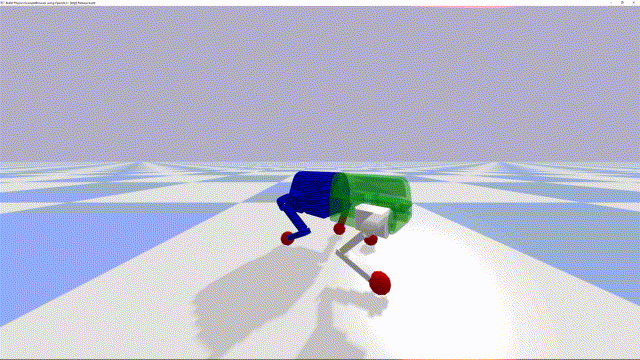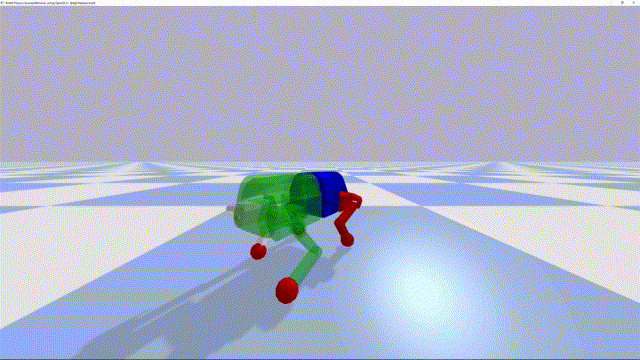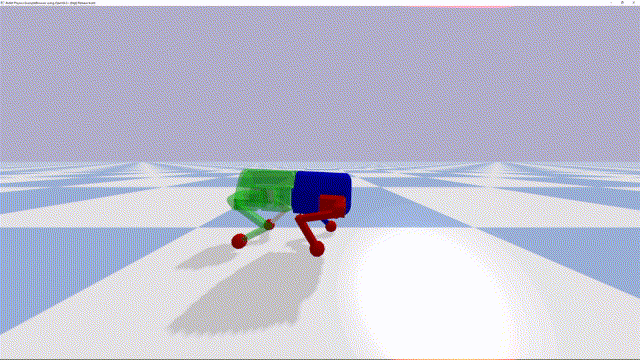
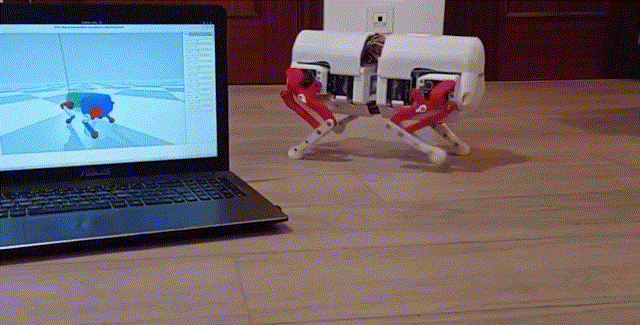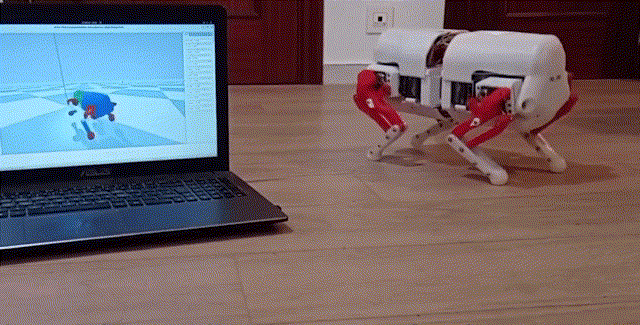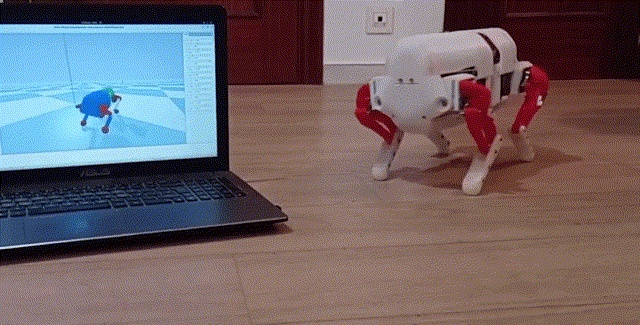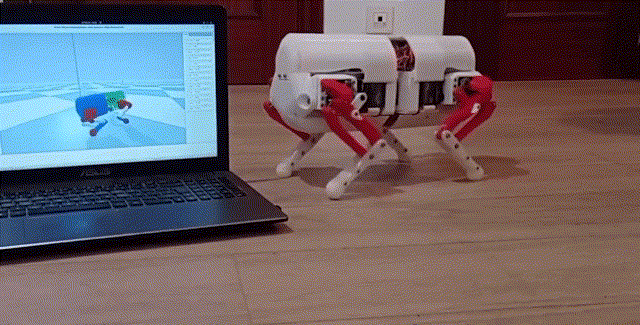
Using a quadrupedal robot to study neural control of turning and steering
A quadrupedal robot "Miguel" was constructed by our collaborator Miguel Ayuso Parrilla
and reproduced in in our group by Drs. Sergey N. Markin, Simon M. Danner, and Ph.D. student Andrew B. Lockhart. The robot will be controlled
by a controller imitating the brainstem-spinal circuits involved in the control of locomotion and steering in mice (see above).
The robot will be used for the analysis and comparative investigation of different algorithms and neural mechanisms of turning and navigation.
Robot simulation
Improving breathing with limb muscle stimulation after cervical SCI
Pennsylvania Department of Health, SAP # 4100089343; 6/01/2021- 5/31/2023
PI: Bezdudnaya T, Co-PI: Rybak IA
The long-term goals of this project are to investigate the organization of spinal respiratory circuits, evaluate conditions for
activation of breathing by stimulation of limb muscle afferents in intact and injured spinal cord, and develop a novel
computational model of the respiratory system. This model will include both supraspinal and spinal levels of respiratory control,
afferent inputs from limb muscles, and reproduce all experimental data. The experiments will be performed in adult decerebrate
rats in vivo in close interaction with computational modeling studies conducted in parallel. The proposed collaborative project
has the following Specific Aims:
Aim1: Investigate ipsi- and contralateral effects of limb muscle afferent stimulation on the respiratory spinal motor circuits,
and on the breathing (airflow) pattern in decerebrate rats in vivo. With a focus on the forelimb muscle (biceps brachii),
we will study the effect of electrical stimulation of ipsi/contralateral muscle afferents on the activity of phrenic
motoneurons, respiratory interneurons, and ventilation. Also, interconnections between phrenic and bicep spinal
networks will be investigated using transsynaptic tracing techniques and immunohistochemistry. Based on the obtained data,
the computational model will be developed and include supraspinal and spinal levels of the respiratory control and
relevant interactions between respiratory networks and non-respiratory muscle.
Aim2: Investigate intraspinal mechanisms and pathways mediating the effects of muscle afferent stimulation on the
respiratory spinal motor circuits following the complete or partial elimination of supraspinal control in C1
transected and C2 hemisected, decerebrate rats in vivo. Partial and complete sections of the cervical spinal
cord will serve as the models of SCI and as comprehensive tools to dissect the intraspinal interconnections
between respiratory and locomotor systems. C1 (cervical segment) transection will be used to completely
remove supraspinal inputs to the spinal circuits and all effects of biceps muscle stimulation on phrenic
motor circuits will be purely intraspinal. Partial sections of the cervical spinal cord at C2 level
eliminates supraspinal control of respiration unilaterally and will be performed to study the effect of
partial removal of supraspinal inputs and left-right interactions of supra- and intraspinal inputs to the
spinal respiratory network using ipsi- and contralateral electrical stimulation of biceps brachii muscle.
The final computational model will be developed using obtained experimental results.
The final model will offer the unique opportunity to assess how cervical SCI can alter respiratory function,
contribute to plasticity and respond to treatments.
Propriopsinal neuron function in normal and post-SCI locomotion
NIH/NINDS R01 NS112304; 03/15/2021 - 01/31/2026
PIs: Danner SM, Magnuson DS, Whittemore SR
Despite the more than 100 years since the recognition of intrinsic spinal locomotor circuits, many of the functional details of those circuits and their contributions to recovery following spinal cord injury (SCI) remain to be determined. Recent development of powerful molecular tools enables functional dissection of neural circuitry via reversibly silencing neurotransmission and trans-synaptic labeling. We will combine these tools with sophisticated gait and kinematic analyses, that includes the full repertoire of speed dependent gaits, to provide the functional and anatomical information necessary for building and refining an advanced neurobiomechanical computer model of the rat spinal cord, body and limbs. We will focus on two classes of spinal cord interneurons, the long ascending (LAPNs) and descending (LDPNs) propriospinal neurons, that interconnect the forelimb and hindlimb circuits and central pattern generators in the two enlargements, and investigate their role in the intact spinal cord and after SCI using both hemisection and contusion models. Our preliminary data show that these LAPNs/LDPNs are essential components involved in speed-dependent gait expression. Silencing these neurons partially decouples the right and left limbs at each girdle. Surprisingly, silencing these neurons after an incomplete contusion injury results in better overground locomotion, a result that is hard to reconcile based on current knowledge and observations in uninjured animals. Using viral-based trans-synaptic labeling we will determine the sensory, descending and propriospinal inputs onto both LAPNs and LDPNs. We will utilize both existing and new physiological and biomechanical data (Aim 1) as well as new anatomical data (Aim 2) to build and refine our computational model (Aim 3). Then, in vivo experiments and computer modeling will be performed in parallel (Aim 4) to determine the roles that ipsilateral and commissural LAPNs and LDPNs play in locomotor behavior, including the full range of locomotor gaits, and in recovered function after hemisection and incomplete contusion injuries. We suggest that a deeper understanding of long propriospinal neurons represents an important step towards the development of new therapeutic tools for recovery after SCI.
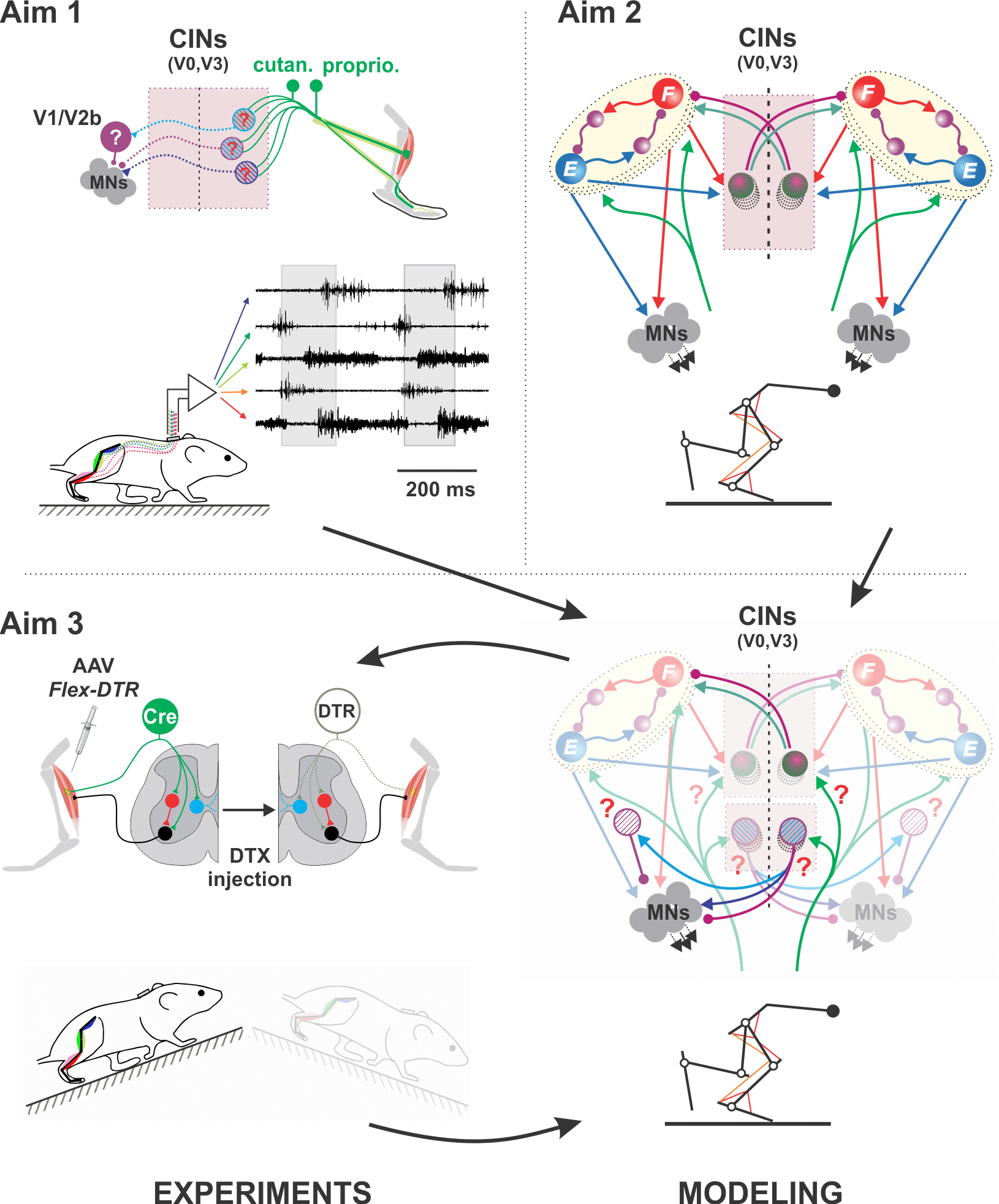
Spinal circuits for sensorimotor integration and interlimb coordination during locomotion
NIH/NINDS R01 NS115900; 09/21/2020 - 06/30/2025
PI: Danner SM, Co-PI: Akay T
Somatosensory feedback from the limbs is essential for locomotion and its recovery after spinal cord injury. To achieve stable locomotion, the spinal cord needs to process afferent feedback signals and properly adjust muscle activation and interlimb coordination. But the interactions of somatosensory feedback with the spinal circuitry during locomotion have yet to be understood on the same level of detail. In this project we propose to address this gap of knowledge by combing mouse genetics, in vivo electrophysiology, and behavioral analyses with computational modeling of spinal circuits and the musculoskeletal system. This multidisciplinary project will be performed in close interactive collaboration between two investigators with strong and complementary expertise in computational (Simon Danner, Drexel University, PI) and experimental studies of neural control of locomotion (Turgay Akay, Dalhousie University, Canada, Co-PI). The project has the following three aims:(1) Delineate the involvement of multiple spinal interneurons in the processing of sensory information and interlimb coordination by studying crossed reflexes at rest and during locomotion; (2) Design a predictive computational model of the spinal locomotor circuitry and its interactions with the mouse musculoskeletal system; (3) Integrate modeling and experimentation to uncover underlying neural mechanisms. The model will be used to derive informative predictions that will then be tested experimentally. In summary, the proposed multidisciplinary approach is based on state-of-art experimental and modeling methods and will provide important and novel insights into the neural organization of the spinal locomotor circuitry responsible for sensorimotor integration and interlimb coordination during locomotion that cannot be obtained by experimentation or modeling alone.
Limb coordination during locomotion before and after spinal cord injury
NIH/NINDS R01 NS110550; 02/15/2020 - 01/31/2025
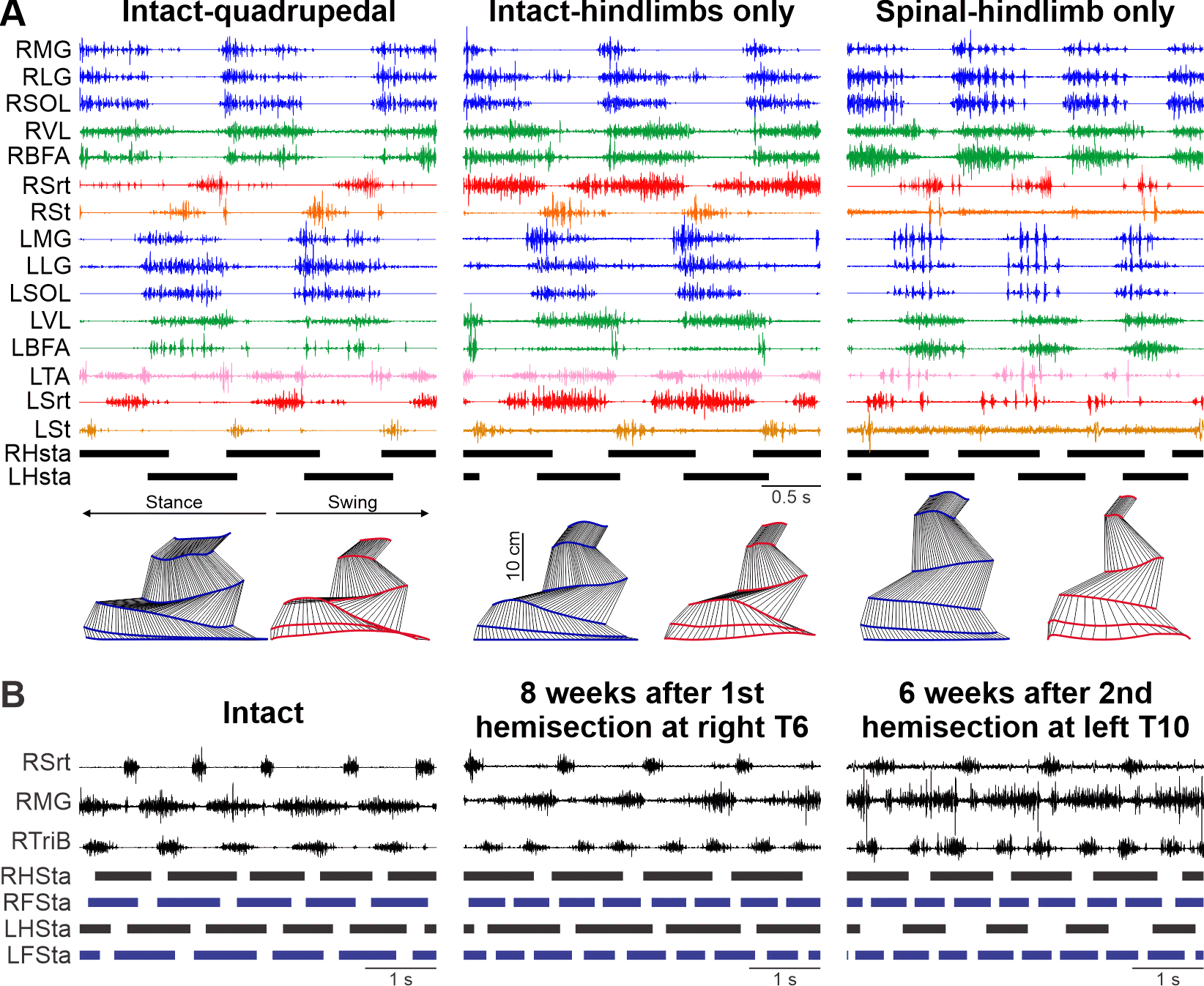
PIs: Rybak IA; Frigon A, Prilutsky BI
Interlimb coordination is essential for maintaining balance when navigating in complex and/or changing environments. It involves complex dynamic interactions between neural circuits at different levels of the nervous system and biomechanical properties of the musculoskeletal system to allow animals to adjust locomotor speed and gait for goal-oriented behaviors. Interactions within the nervous system include those between the spinal circuits controlling each limb, supraspinal inputs and sensory feedback from the limbs. Despite their obvious importance, the mechanisms that control interlimb coordination and contribute to locomotor recovery following SCI and other neurological disorders remain poorly understood. To address this gap in knowledge, we will integrate multiple experimental and modeling approaches to investigate the neural and biomechanical mechanisms controlling interlimb coordination in a feline model before and after SCI disrupting neural communication between the brain and spinal cord and/or between the circuits controlling fore- and hindlimb movements.
The project will be performed in close interactive collaboration between three groups of investigators with strong and complementary expertise in the
experimental study of cat locomotion, including SCI models (Alain Frigon,
Universite de Sherbrooke, Canada), biomechanics of cat locomotion (
Boris Prilutsky, Georgia Tech)
and neural control of locomotion (Ilya Rybak, Drexel University).
Results obtained from these studies will have an important theoretical impact on our understanding of how the limbs are
coordinated during locomotion and how this coordination is altered and adjusted after disruption of spinal
pathways between left-right or cervical-lumbar circuits. The results will identify neural pathways and biomechanical
mechanisms that could be targeted to improve interlimb coordination in people with various movement disorders,
such as SCI, stroke, and Parkinson's disease.
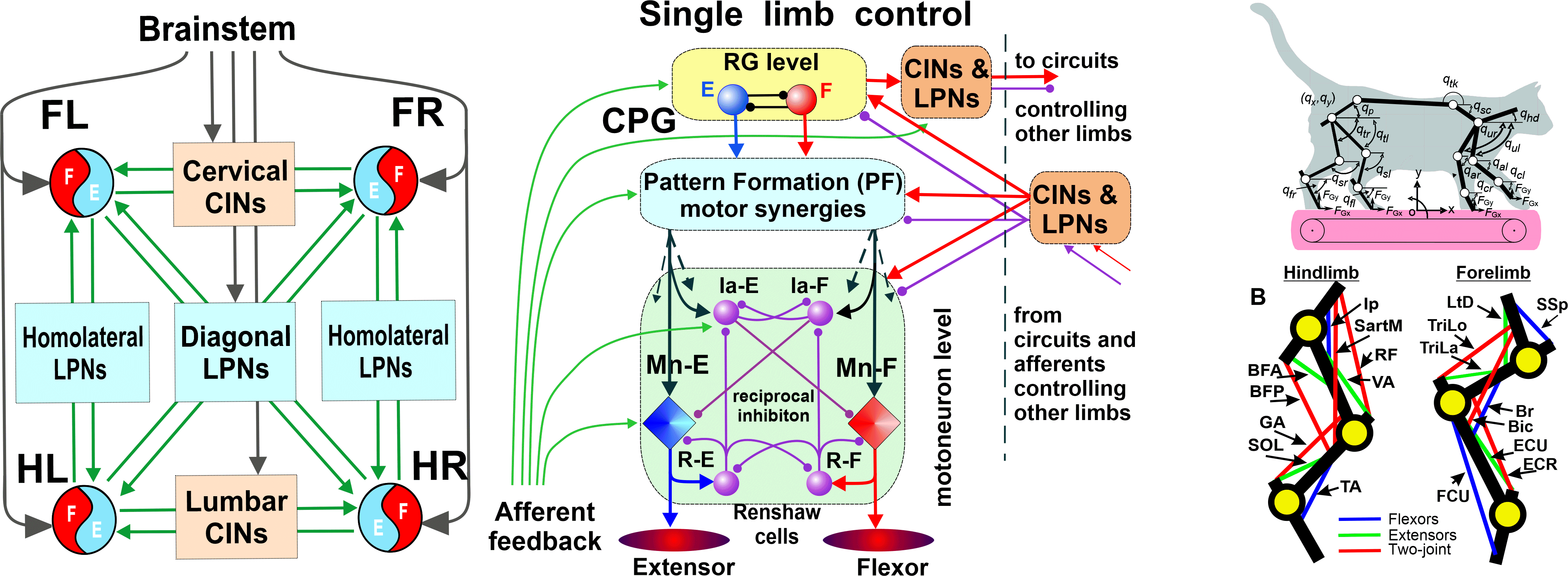
Completed Projects
Neural mechanisms of locomotion evoked by epidural stimulation of the spinal cord
NIH/NINDS R01 NS100928; 07/15/2017 - 05/31/2022
PI: Prilutsky BI; Co-PIs: Markin SN, Deliagina TG, Musienko PE
The overall goal of this project is to determine the contribution of motion-dependent
afferent pathways, selected ascending and descending pathways in the spinocerebellar loop,
and the central pattern generator (CPG) circuitry to the generation of the distinct kinematic and
muscle synergies during normal walking and epidural stimulation(ES)-activated walking in cats with intact and partially
transected spinal cord. This goal will be accomplished in experimental and neuromechanical
computational studies performed in close collaboration among 4 research groups from Georgia Institute of Technology (Prilutsky);
Karolinska Institute, Sweden (Delyagina); Drexel University (Markin) and Pavlov Institute of Physiology, Russia (Musienko).
We anticipate that this study will improve our understanding of how ES,
sensory and supraspinal inputs, and CPG contribute to kinematic and
muscle synergies during locomotion and thus will provide scientific basis for
improvement of ES- stimulation therapies.
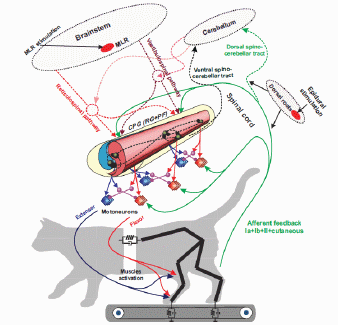
CRCNS: Rhythm generation in rodent spinal cord
NIH/NINDS R01 NS095366; 08/01/2015 - 07/31/2020
PI: Dougherty KJ, Co-PI: Shevtsova NA
The overall goal of this project is to determine the contribution of motion-dependent afferent pathways, selected ascending and descending pathways in the spinocerebellar loop, and the central pattern generator (CPG) circuitry to the generation of the distinct kinematic and muscle synergies during normal walking and epidural stimulation(ES)-activated walking in cats with intact and partially transected spinal cord. This goal will be accomplished in experimental and neuromechanical computational studies performed in close collaboration among 4 research groups from Georgia Institute of Technology (Prilutsky); Karolinska Institute, Sweden (Delyagina); Drexel University (Markin) and Pavlov Institute of Physiology, Russia (Musienko). We anticipate that this study will improve our understanding of how ES, sensory and supraspinal inputs, and CPG contribute to kinematic and muscle synergies during locomotion and thus will provide scientific basis for improvement of ES- stimulation therapies.

PI: Dougherty KJ, Co-PI: Shevtsova NA
The overall goal of this collaborative project is to use state-of-art genetic, molecular, and physiological studies of spinal neurons and neural circuits in combination with computational modeling to dissect the organization and operating mechanisms of the spinal locomotor central pattern generator (CPG). As demonstrated in rodents and cats, activation of the spinal locomotor CPG leads to the restoration of locomotion after upper spinal cord injury. The proposed study will provide an important theoretical basis for the future development of new, effective methods for restoring locomotor function after spinal cord injury and various degenerative disorders affecting normal locomotion.The central issues addressed in this study include understanding the rhythm-generating mechanisms operating in the spinal cord as well as the organization of flexor-extensor interactions.
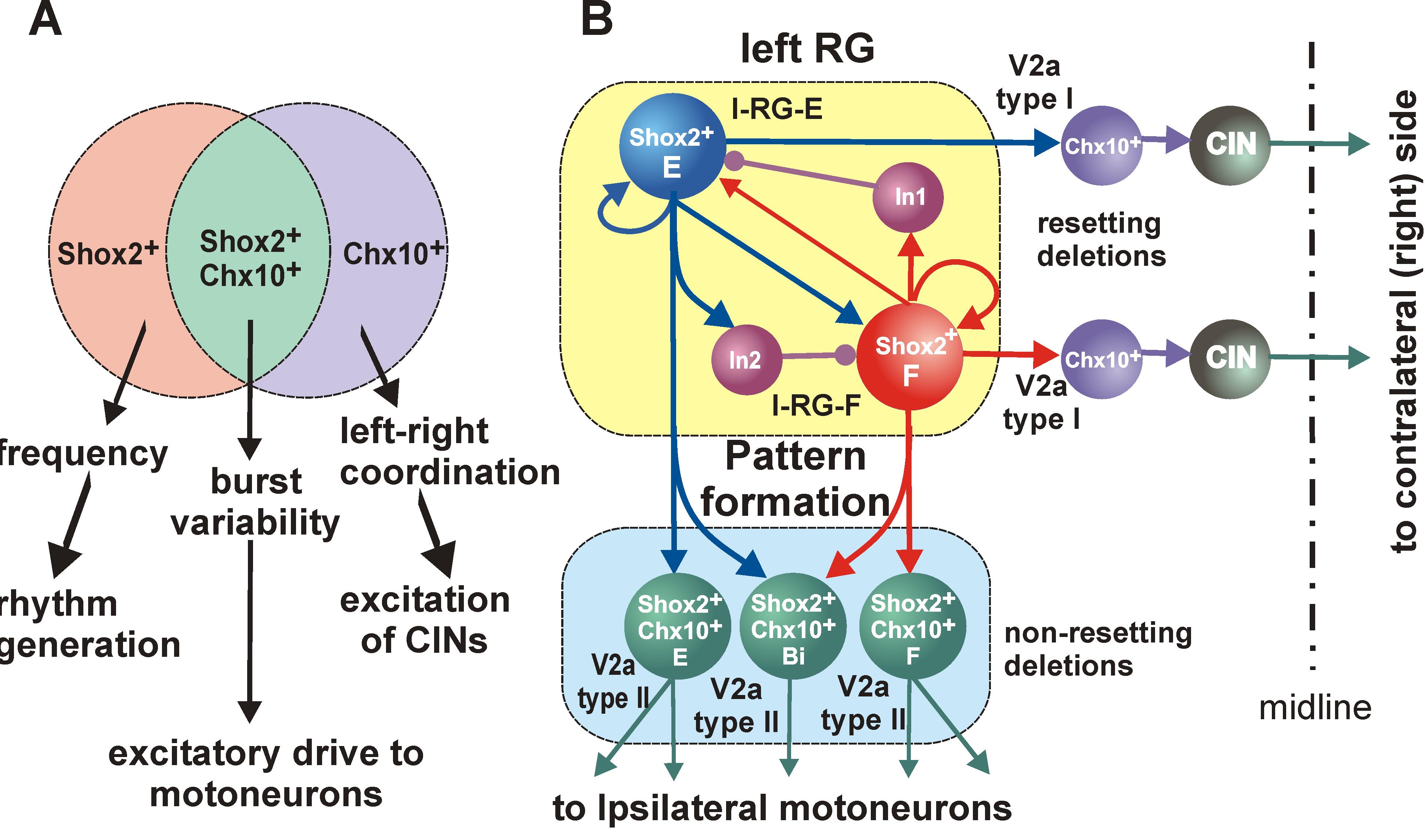
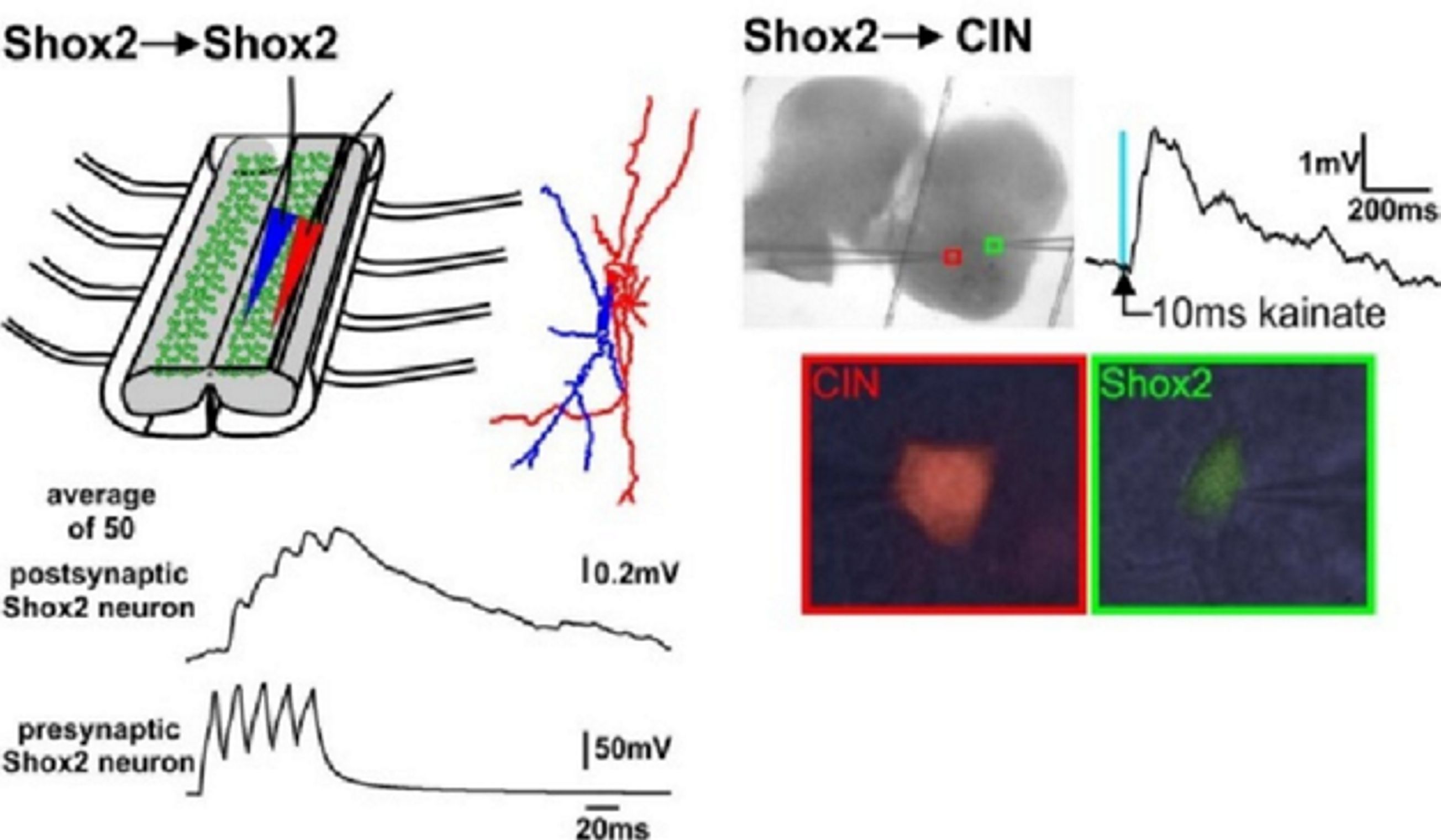
We propose to (a) investigate recently identified spinal interneurons that are considered to belong to rhythm-generating circuits, (b) identify their connectivity pattern, and (c) determine the functional links between these neurons and other key components in the spinal locomotor CPG. The project brings together two laboratories with complementary expertise in experimental and computational neuroscience to: (1) Determine and investigate the cellular basis of rhythmic bursting in Shox2 neurons and their mutual interactions. Investigate potential differences between these neurons with flexor- vs. extensor-related activity. (2) Investigate inhibitory interactions between the flexor- and extensor-related rhythm-generating neurons and the role of genetically-identified V2b and V1 neurons in these interactions. (3) Investigate properties of neuronal and network organization leading to the frequency-dependent flexor-extensor asymmetry and study interactions between flexor-extensor and left-right coordinating networks. The model will be progressively developed by continuous interaction with the experimental studies and will serve both as a testbed for working concepts on the organization of the rhythm generating circuits in the spinal cord and as a source of predictions for subsequent experimental validation.
Spinal cord neural circuits for left-right and flexor-extensor coordination
NIH/NINDS R01 NS090919; 09/15/2014 - 07/31/2020
PIs: Rybak IA, Goulding M, Kiehn O
The overall goal of this project is to dissect the organization of the spinal CPG with focus on the organization of flexor-extensor alternating and left-right coordinating circuits. All work will be done on identifiable classes of spinal interneurons labeled by genetic markers in transgenic mice and/or classified by anatomic or electrophysiological labeling needed to obtain a unified picture of the CPG organization. We propose to identify the functional connectome and the interactions between these fundamental components of the CPG. The experiments performed will use a combination of electrophysiological, imaging, and molecular biology techniques complemented with advanced computer modeling. The three PIs involved in this project have strong background and experience in spinal cord studies and unique expertise in physiological, genetic and molecular methods (Martyn Goulding, Salk Institute, and Ole Kiehn, Karolinska Institute, Sweden) and computational modeling (Ilya Rybak). The Specific Aims of the project include: investigation of activity patterns and connectivity of the genetically identified spinal interneurons responsible for left-right coordination during locomotion (Aim 1), investigation of the genetically identified spinal interneurons and their connectivity responsible for flexor-extensor alternation and their interactions with the circuits providing left-right coordination (Aim 2), development of a comprehensive computational model of spinal cord circuits (Aim 3). The proposed multidisciplinary approach based on the state-of-art methods and close collaboration between the three leading labs will investigate and analyze the specific contributions of left-right and flexor-extensor coordinating neuronal circuits and their interactions to the generation and control of the locomotor pattern and provide important insights into the neural organization of the mammalian spinal cord, leading to new strategies to treat spinal cord injury and degeneration spinal cord disorders.
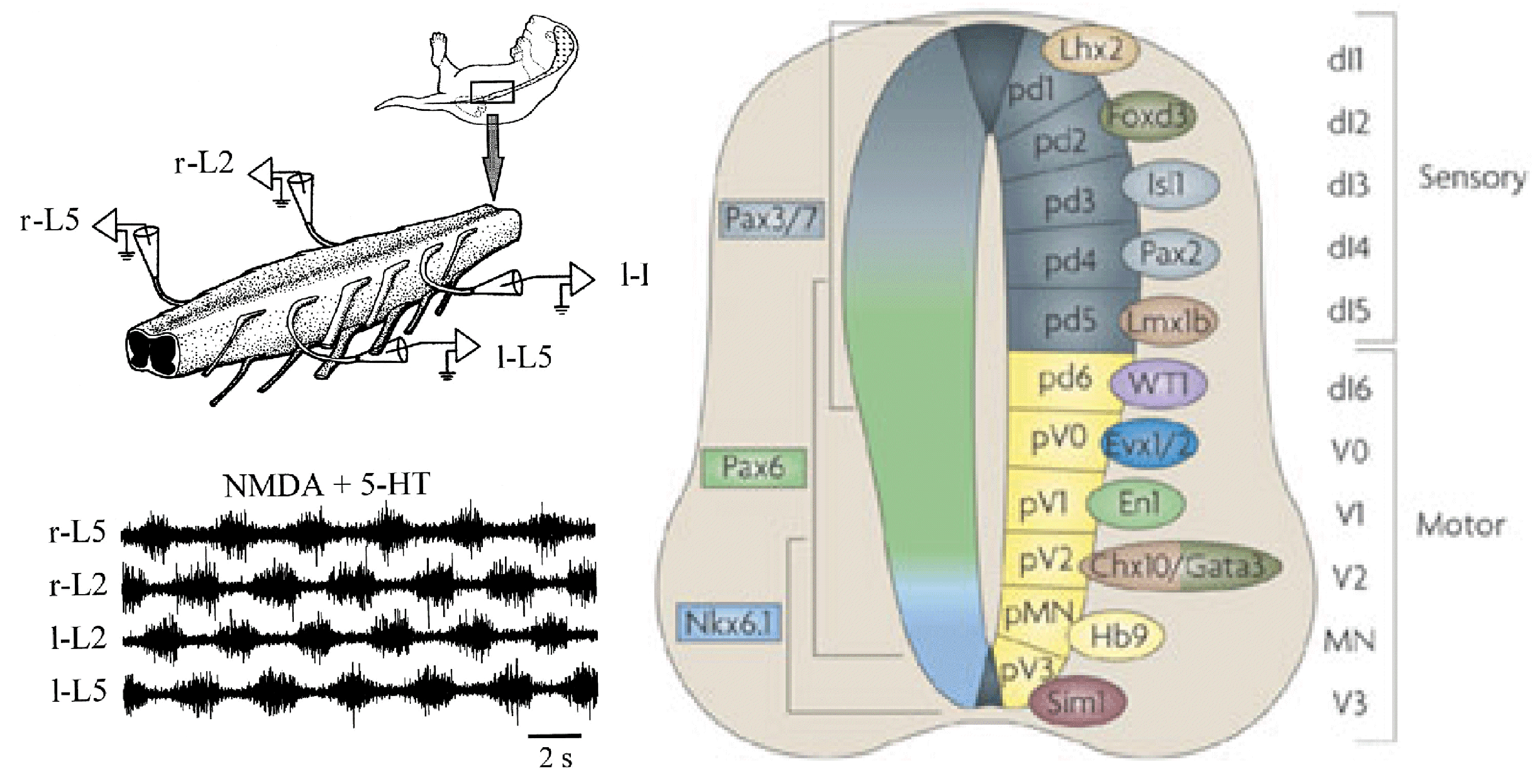
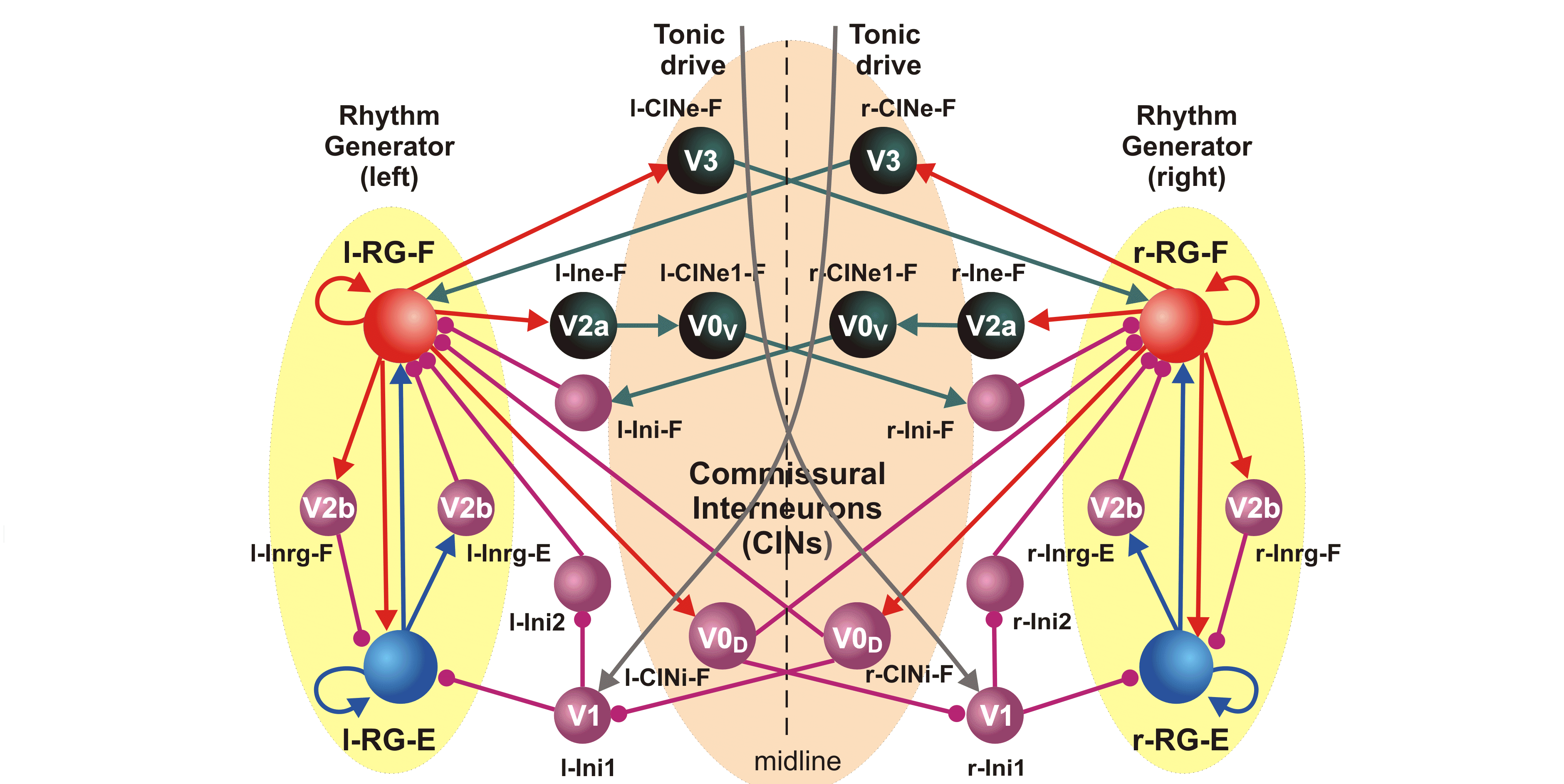
CRCNS: Organization of locomotor CPG in the rodent spinal cord
NIH/NINDS R01 NS081713; 07/01/2012 - 06/30/2017
PIs: Harris-Warrick RM, Rybak IA
This project combines electrophysiological and modeling approaches to study the organization and neuronal composition of the Central Pattern Generator (CPG) neural circuits in the mammalian spinal cord that coordinate rhythmic neural activity driving locomotion. This study will take a general approach that utilizes various spontaneous and evoked perturbations in the locomotor pattern (including deletions of motoneuron activity, spinal cord lesions and pharmacological manipulations) as probes to understand CPG organization and function. It is proposed that analysis of the influences of these perturbations on the rhythmic motor pattern and the activity of identified spinal interneurons will provide important insights on the spinal CPG organization and operation. These data will be used to develop a comprehensive computational model of the spinal locomotor CPG, and to refine and validate this model, so that it can reproduce both normal locomotor activity and the consequences of experimental perturbations. In predictions to guide subsequent experimental investigations. The project brings together two senior scientists with complementary and overlapping expertise in experimental (Dr. Harris-Warrick at Cornell University) and computational (Dr. Rybak at Drexel University) neuroscience. It has three interlocking objectives: 1) Explore alterations in behavior of, and synaptic drive to, motoneurons and genetically defined interneurons during spontaneous and evoked deletions in flexor and/or extensor rhythmic motor activity, to define the possible function of these interneurons in the locomotor CPG. 2) Explore the consequences of reducing CPG complexity by spinal cord hemisection and removal of spinal segments, and compare the behavior of identified interneurons in the reduced cord during deletions and after pharmacological blockade of synaptic inhibition. 3) Develop a comprehensive computational model of the neural circuits forming the locomotor CPG in the neonatal mouse spinal cord that includes genetically identified interneurons and suggests their roles in the generation of the locomotor pattern. Validate this model in simulations reproducing the specific transformations in motoneuron and interneuron activity and the entire locomotor pattern during experimental perturbations proposed in objectives 1 and 2.The model will be progressively developed by continuous interaction with the experimental studies, and will serve both as a testbed for working concepts on spinal cord organization and as a source of predictions for subsequent experimental validation. Better understanding of the organization and function of the spinal CPG will provide essential insights into future clinical strategies for restoration of locomotor function after spinal cord injury.
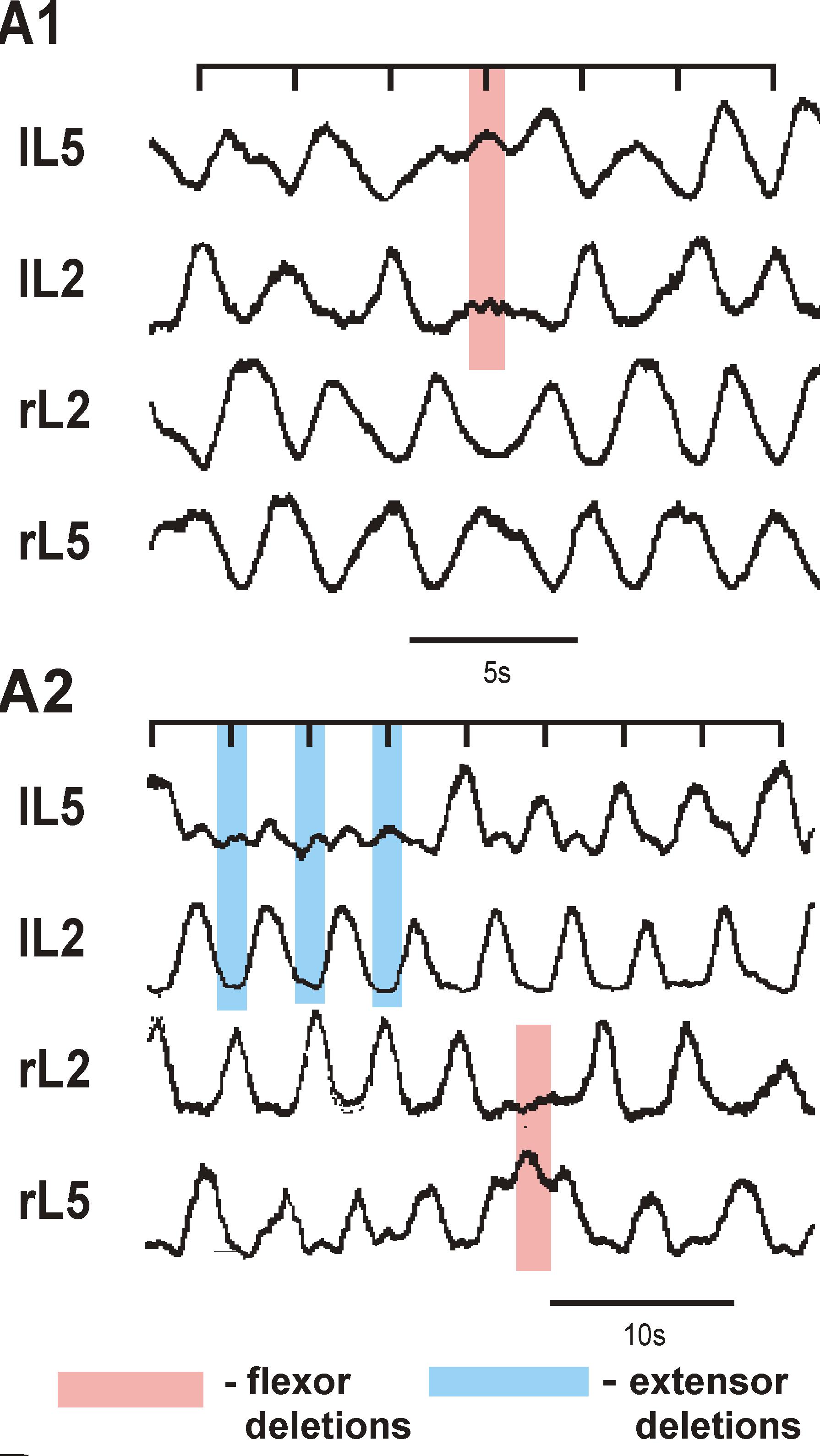
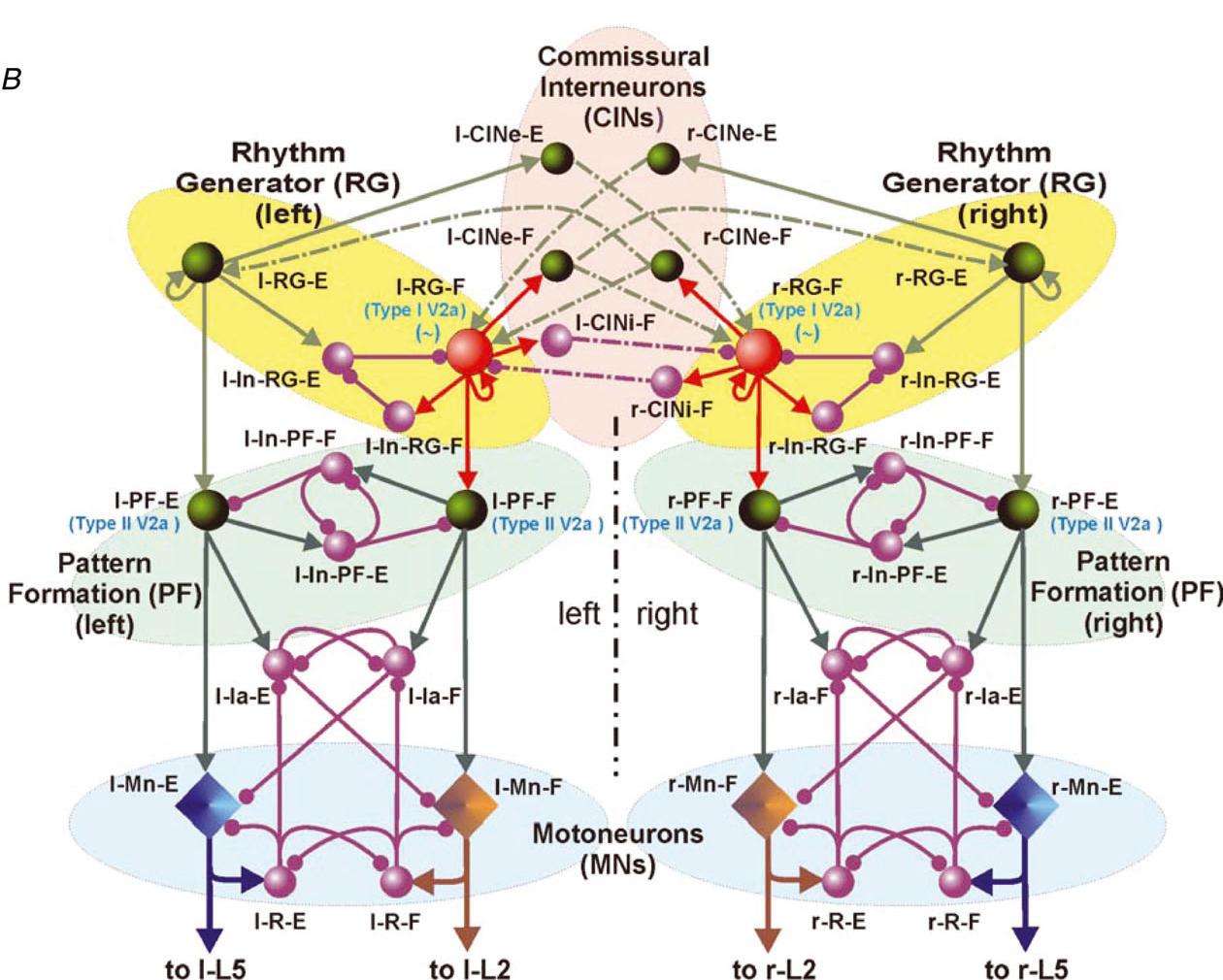
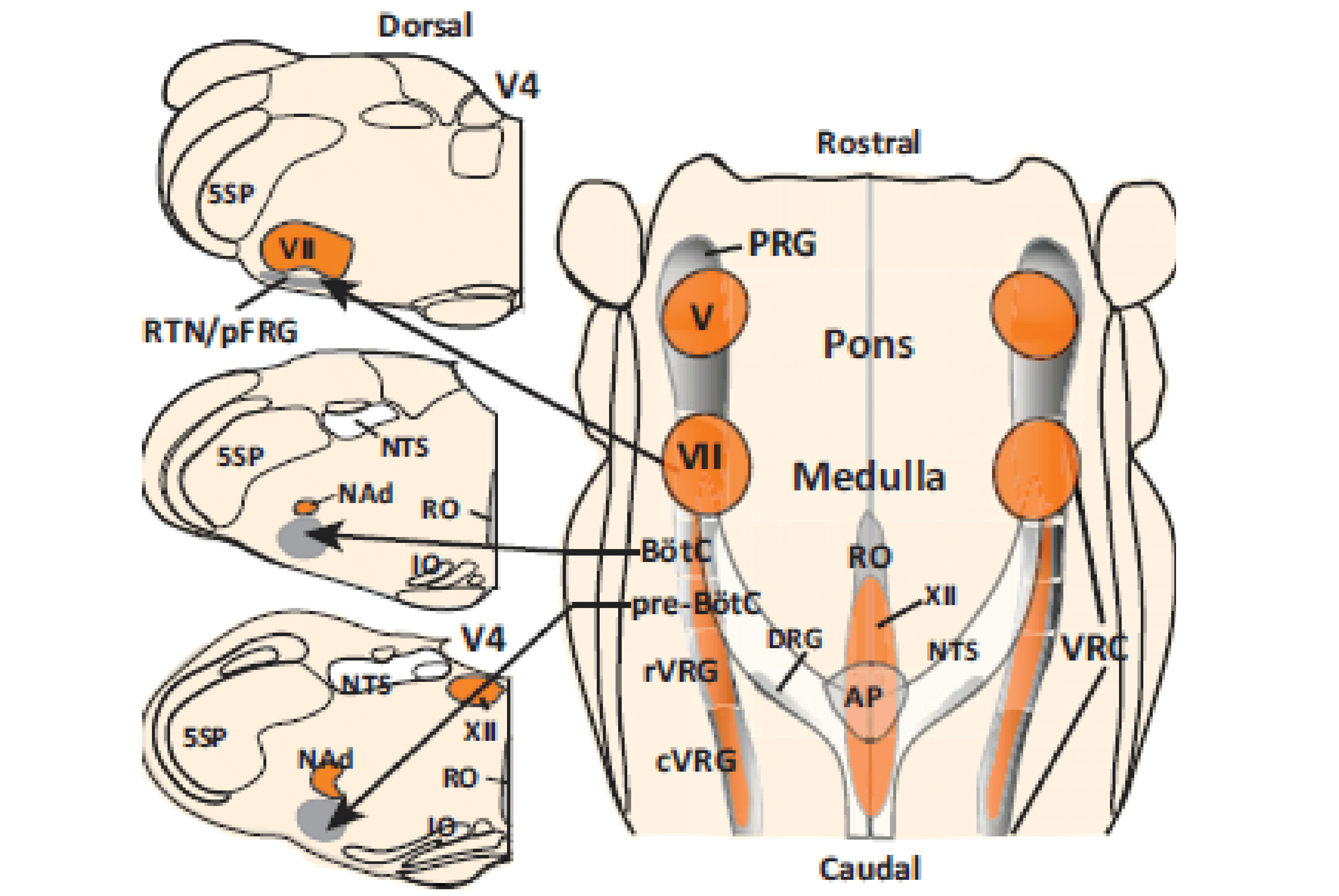
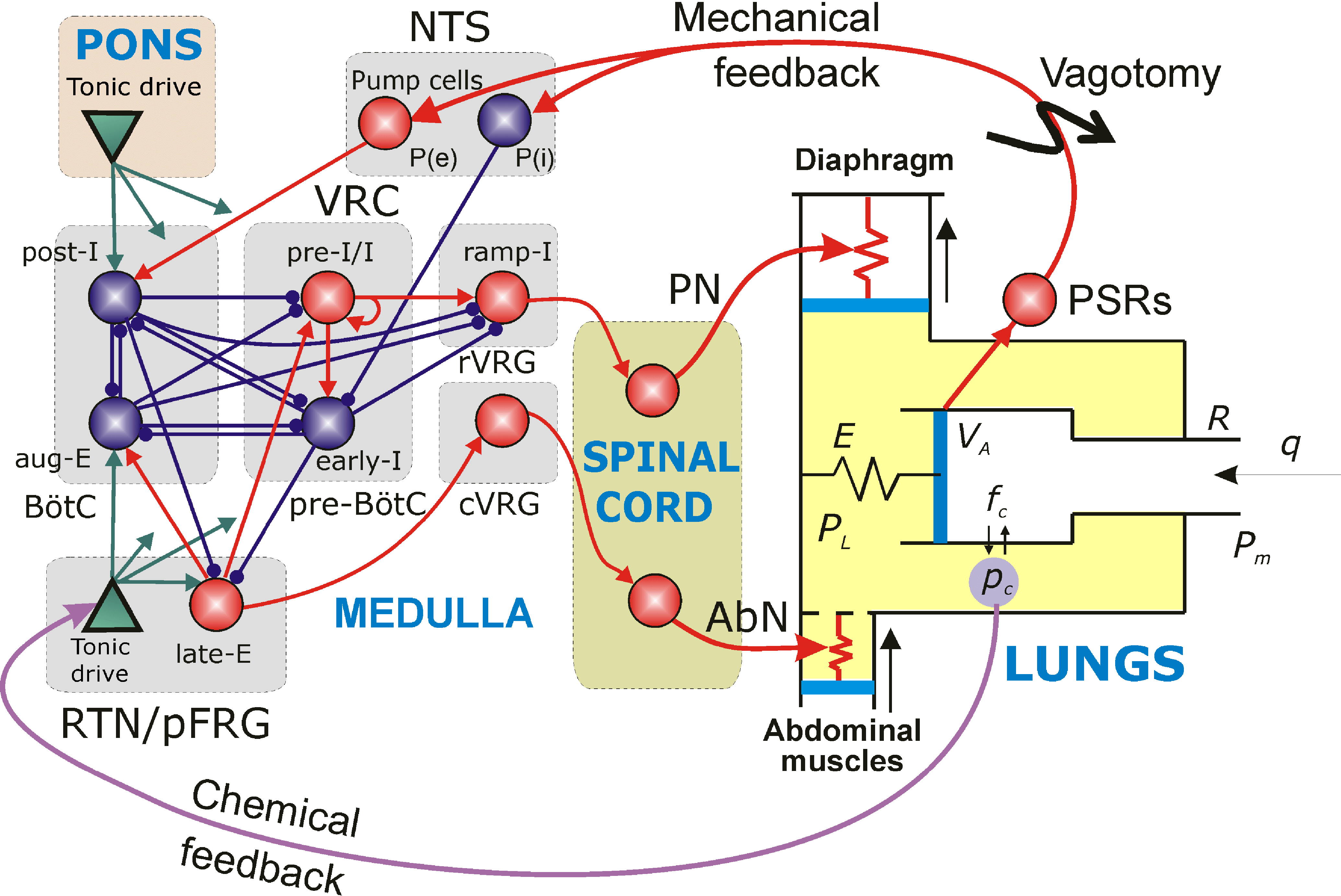
Multiscale model of neural control of breathing
NIH/NINDS R01 NS069220; 09/01/2010 - 08/30/2017
PIs: Rybak IA, Ben-Tal A, Dick TE, Paton JFR, Smith JC
The specific aims of this multi-institutional project are: (1) develop a Physiome-type, predictive, multiscale computational model of neural control of breathing that links multiple physiological mechanisms and processes involved in the vital function of breathing but operating at different scales of functional and structural organization, (2) validate this model in a series of complementary experimental investigations and (3) use the model as a computational framework for formulating predictions about possible sources and mechanisms of respiratory pattern alteration associated with heart failure. The project brings together a multidisciplinary team of scientists with long standing collaboration and complementary expertise in respiration physiology, neuroscience and translational medical studies (Thomas E. Dick, Case Western Reserve University;Julian F.R. Paton, University of Bristol, UK; Robert F. Rogers, Drexel University; Jeffrey C. Smith, NINDS, NIH, intramural), mathematics, system analysis and bioengineering (Alona Ben-Tal, Massey University, NZ), and computational neuroscience and neural control (Ilya A. Rybak, Drexel University). The end result of our proposed cross-disciplinary modeling and experimental studies will be the development and implementation of a new, fully operational, multiscale model of the integrated neurophysiological control system for breathing based on the current state of physiological knowledge. This model can then be used as a computational framework for formulating predictions about possible neural mechanisms of respiratory diseases and suggesting possible treatments.
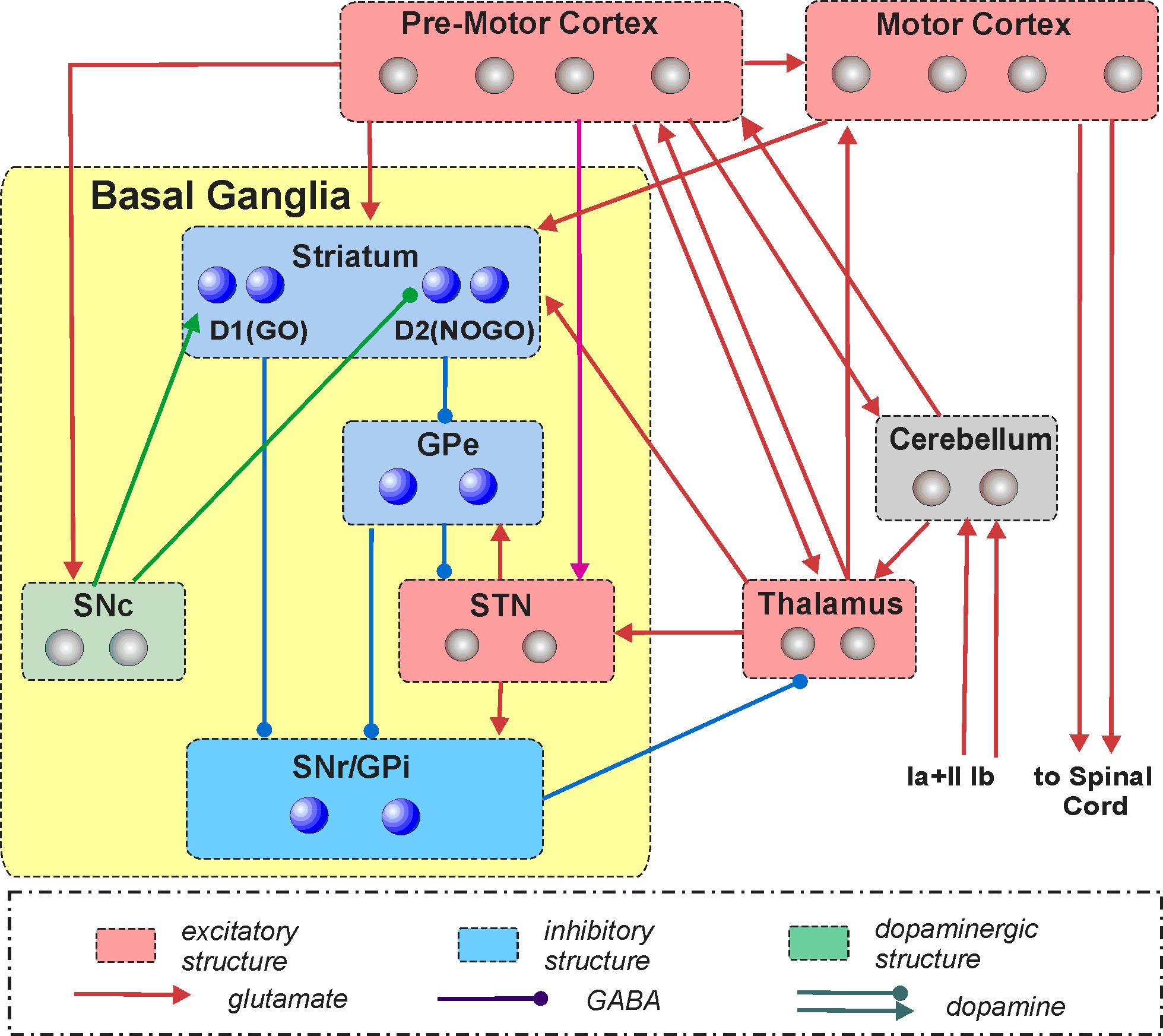
Computational modeling of the primate motor control system for subsequent simulation studies of Huntington's disease
CHDI Foundation; 10/01/2014 - 09/30/2017
PI: Rybak, IA
This project represents the first steps towards the ultimate goal of creating a comprehensive multi-level, multi-scale neural model of the motor control system. The model will ultimately serve as a test bed for studying motor dysfunctions associated with and/or resulted from Huntington's disease (HD). We will take a multi-resolution modeling approach in which more detailed cellular and network neuron models (representing various CNS regions) will be sequentially incorporated into a larger systems-level model that produces simulated movements. The initial low-resolution systems-level model will describe system interactions between the major brain structures (nuclei, represented as model compartments) involved in planning, learning, and control of individual and successive arm reaching movements. We will consider (simulate and investigate) multiple interrelated motor/behavioral processes involving the basal ganglia (BG) including goal-directed reaching movements, decision-making between competing motor strategies, and motor task execution. The resultant model will provide a test bed for subsequent simulations of sequential reaching-related motor dysfunctions associated with HD, as well as for predictions of the possible effects of HD treatments at various functional levels.
2010-2015
CRCNS: Role of sensory feedback in locomotor recovery after spinal cord injury
NIH/NIBIB R01 EB012855
PIs: Lemay MA, Markin SN, Prilutsky BI
2008-2013
Modeling of pathogenic breathing pattern dysregulation in cardiopulmonary disease
NIH/NHLBI R33 HL087379
PI: Rybak IA
2006-2013
CRCNS: State dependent neural mechanisms for respiratory pattern generation
NIH/NINDS R01 NS057815
PIs: Rybak IA, Smith, Paton JFR
2006-2013
Spinal control of locomotion: studies and applications
NIH/NINDS; R01 NS048844
PIs: Rybak IA, McCrea DA, Prilutsky BI, Lemay MA
2005-2006
Modeling state-dependent neural mechanisms for generation of the respiratory rhythm and pattern
NIH/NINDS Research Contract
PI: Rybak IA
2002-2007
Computational studies of the respiratory brainstem
NIH/NINDS R01 NS046062
PIs: Lindsey BG, Rybak IA, Orem J, Dick TE, Solomon IC
2002-2007
The PreBotzinger circuit and respiratory rhythm generation
NIH/NHLBI R01 HL072415
PIs: McCrimmon DR, Rybak IA (Drexel subcontract)
2002-2005
Closed-loop brain-machine interface for augmenting motor performance
DARPA
PIs: Nicolelis M, Chapin JK, Rybak IA (Drexel subcontract)
2002-2003
Development of biomimetic CPG-based neural controller for bipedal robot locomotion
ONR N000140210086
PI: Rybak IA
2001-2004
Modeling the brainstem neural mechanisms for the respiratory pattern generation
NSF 0091942
PI: Rybak IA
2000-2001
Computational modeling of spinal cord neural circuitry for limb control during locomotion
ONR N000140010719
PI: Rybak IA
1999-2003
Development of biomimetic robots and sensors using hybrid brain-machine technology
DARPA
PI: Chapin JK, Rybak IA (Drexel subcontract)
A model of attention-guided visual perception and recognition
Past and Present Funding (NIH, NSF)

© Ilya Rybak. Last updated 10/02/2022